Abstract
Background: Microglia, the innate immune cells of the central nervous system (CNS), originate from erythromyeloid progenitor cells in the embryonic yolk sac. As the innate immune cells of the CNS, microglia play an important role in innate immunity, neurogenesis, and brain tissue repair by activating the alpha7 nicotinic acetylcholine receptor (α7nAChR). They are the key target cells for HIV-1 infection in the CNS and have been shown to produce a chronic inflammatory response in the brain which underlies HIV-associated neurocognitive disorders (HAND). Apart from the microglial neuroinflammation, recent studies have reported that activated microglia could induce neuronal apoptosis through the release of neurotoxic factors (e.g., ROS and NLRP3 inflammasome) in HAND. In addition, α7nAChR was significantly up-regulated in the striatum which is the most serious part of neuron apoptosis in gp120 transgenic mice brains. However, the underlying mechanism of neuronal cell apoptosis by gp120-activated microglia remains unclear. Here, this study aimed to explore the mechanism by which HIV-1 gp120-activated microglia induce neuronal apoptosis.
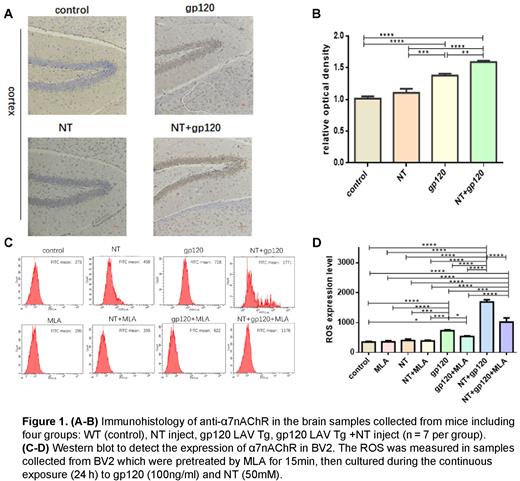
Materials and methods: The expression of α7nAChR was examined by immunohistochemistry in the brain of HIV-1 LAV gp120 transgenic (Tg) mice (C57BL/6J background, 12-month-old). Methyllycaconitine (MLA, specific α7nAChR agonists) and Nicotine (NT, specific α7nAChR antagonists) were used to evaluate the effect of α7nAChR on apoptosis induced by gp120. Immortalized microglial cells (BV2) were incubated for 24 hours with gp120, NT, and gp120+NT, respectively, with or without preincubation with MLA for 15 minutes to obtain their supernatants. Next, the neuronal cells (SH-SY5Y) were treated with the same as BV2 or with collected BV2 culture supernatants. The apoptosis effect of SH-SY5Y and the level of ROS in the BV2 culture supernatants were assessed via quantitative analysis of flow cytometry. Comparisons between supernatant groups and drug groups were conducted to evaluate the effects of the SH-SY5Y apoptosis induced by microglia. Western blotting of microglia cell lysates was employed for the analysis of cellular signaling pathways. Student's t-tests and ANOVA were applied to determine statistical significance.
Results: The expression of α7nAChR in HIV-1 gp120 transgenic mice was significantly upregulated (P < 0.001). The effect of apoptosis was more significant in microglia culture supernatants groups compared to that in drug-treated groups (P < 0.001). Neuronal activity was not changed in the NT group or MLA group than in the control, but HIV-1 gp120 could increase the number of apoptotic neurons (P < 0.01). Significant neuronal apoptosis was observed when treated with BV2 supernatants from the group treated by combining the gp120 with NT (P < 0.001), and this effect was in turn inhibited by MLA (P < 0.001). These results suggested that gp120-activated microglia might induce neuronal apoptosis invitro owing to gp120 upregulating α7nAChR. In addition, the ROS level in microglia was significantly upregulated following gp120 exposure (P < 0.001), and this effect could be enhanced by NT (P < 0.001). Notably, there was a positive correlation between the levels of ROS in microglia culture supernatants and the levels of neuronal apoptosis treated in the same supernatants. This positive relevance indicated that microglia might induce neuronal apoptosis by producing excessive ROS. The expressions of p53, OMI, and caspase-3 significantly upregulated following gp120 exposure with the down-regulate expression of XIAP (P < 0.001). And this effect could be enhanced by NT (P < 0.001). The results of western blotting indicated that gp120 and NT can up-regulate the ROS to activate p53 by α7nAChR, which could lead to an increased OMI and decreased XIAP levels. (P < 0.001).
Conclusions: Microglia may play a critical role in regulating neuronal apoptosis in HAND. HIV-1 gp120 and NT could increase the generation of ROS through α7nAChR in microglia, which leads to neuronal apoptosis. Moreover, our results showed that the combined action of NT and gp120 may upregulate p53 and Omi / HtrA2 protein expression, and the downregulation of XIAP protein may significantly activate the level of caspase-3. (Acknowledgements:Corresponding author: Hong Cao, gzhcao@smu.edu.cn; National Natural Science Foundation of China, No. 82172259 to H.C.)
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal